pHlocrite™ is a small independent company based in the south of the UK. We manufacture and supply synthetic semi-calcined dolomitic limestone specifically for:

AUTOMATIC pH CORRECTION - pHlocrite™ is a synthetic high magnesia, semi calcined dolomitic limestone filter medium for the removal of carbon dioxide, iron and manganese from water. It also provides automatic pH control and adjustment of the Langelier Saturation Index, establishing well balanced water through simple filtration.

SELF-REGULATING - pHlocrite™ dolomitic filter medium acts both chemically and physically raising the pH, hardness (calcium & magnesium) and alkalinity, and diminishing acidity (excessive carbon dioxide) until the point of saturation is established (SI=0). The treated water deposits a highly protective, complex and dense 'rust lime' film which protects ferrous pipes and tanks against corrosion and cuts contamination by lead, zinc and copper. Also, due to the irregular shape of the porous grains, pHlocrite™ provides maximum surface area per unit volume which improves the removal of turbid matter.
HEAVY METAL REMOVAL is achieved due to the alkaline milieu on the surface of the pHlocrite™. Metallic ions (if present) are trapped in the form of their insoluble hydroxides including lead, cadmium, copper and zinc.
GRADES - The advantage of our synthetic dolomite mix over the natural product is that pHlocrite™ results in optimum performance through positive and precise pH adjustment. All grades are very competitively priced and extremely economical to use, an additional bonus being the lower running costs of the magnesia grades which extract more carbon dioxide per unit weight. It requires no dosing equipment and fits into any gravity or pressure filter tank, placed as a layer on top of a conventional sand bed.
Basic Langelier Saturation Index Calculator
Once you have measured the various parameters below, you can use this convenient Langelier Saturation Index calculator to determine whether your water is corrosive, scale forming or nicely balanced. If the water is corrosive, the water will try to saturate itself by dissolving the surface composition structure of your pool. If it is scale forming it will try to correct its over saturation by precipitating the hard water salts causing cloudy water and eventually deposit scale on pool components
In the above formula:
Calcium Hardness factor - Optimum range 75 to 150 mg/liter
Total Alkalinity factor - Optimum range 100 to 150 mg/liter
Suggested temperature - between 27 and 29.5 Degrees C in pools
pH Optimum range - 7.2 to 7.6
If the result of this calculation is zero, the target goal, then the water is balanced. If the answer is less than 0, the water leans toward being corrosive. If it is greater than 0, then it is indicative of scale forming. An acceptable range is -0.5 to +0.5, with the optimum goal of 0 (zero). If the result is outside this range, adjustments should be made to the hardness, alkalinity and pH to bring it as close to zero as possible and ideally within the optimum ranges shown above.
Be aware however, that while the water may be balanced from a corrosion and scaling point of view it does not necessarily mean that it is ideal for chemical efficiency and bather comfort.
Calculation of Free Carbon Dioxide
The free carbon dioxide (CO2) content of any water may be calculated using the following formula if the pH and alkalinity are known.
A typical soft water with an alkalinity of 20mg/l (as CaCO3) and a pH of 6.1 would therefore have a free carbon dioxide content of :-
6.2878 + 1.301 - 6.1 = 1.4888
Antilog10 of 1.4888 = 30.8 mg/l CO2
Some Handy Tech Information Sheets
Here are some of our technical notes that you might find useful.
A Guide to the Chemistry of pHlocrite™
Brief Technical Specifications
Appearance: Porous Granules - 2 - 4.5mm (4/8 BS Mesh)
Chemical Name: Semi-calcined dolomitic limestone (MgO CaCO3)
Filtration Rate: 10-30 metres an hour
Bulk Density: 1,100 kilos per cubic metre (depending on grade)
Hardening: In return for every 10 milligrams per litre carbon dioxide (CO2) alkalinity and total hardness will be raised by approximately 16 milligrams per litre as calcium carbonate (CaCO3)
Consumption: The appropriate specific amounts are a function of the chemical and physical data of the water in question. Generally, however . .
Ground Water - 200 kilograms per cubic metre per hour
Distillates - 90 kilograms per cubic metre per hour
Swimming Pools - 20 kilograms per cubic metre per hour
Backwashing: All methods applied to conventional sand filters may be used for back-washing, including combined air/water scouring.
pHlocrite™ and the Dolomite Process
pHlocrite™ dolomitic filter material is characterised by the following physical and chemical properties:
Outer appearance: white and grey angular porous grains
Granulation: 2 - 4.5mm (4/8 BS Mesh)
Apparent density: approx. 170 lb/ft3 (2.72 g/ml)
Bulk Density: approx. 72 lb/ft3 (1.15 g/ml)
Hardness (Mohs' scale): 3.5 - 4.0
pHlocrite™ synthetic dolomitic filter medium is a combination of magnesium carbonate, calcined to convert it to the oxide (MgO), and high purity calcium carbonate (CaCO3), sieved to appropriate granulation.
It is a special feature of the pHlocrite™ manufacturing process that the calcining temperature is controlled to acheive the desired degree of 'hardness' of the MgO without producing the undesirable free lime or lack of MgO conversion that can result from straying from the critical calcining temperature required for the natural dolomites which tend to contain around 0.2% of free lime even when calcined at their optimum conversion temperature.
The reaction of pHlocrite™ is alkaline and therefore able to neutralise the excessive portion of free CO2 according to the following equation :-

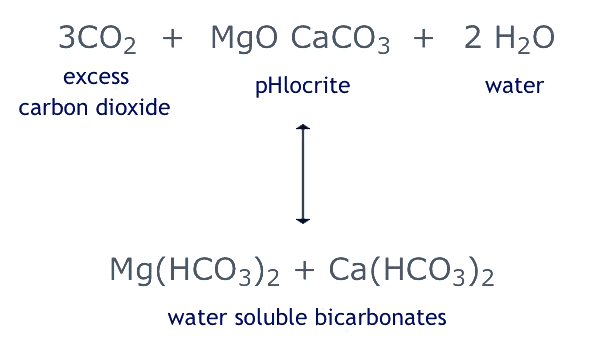
The overall result is that the excess free CO2 (if present) is chemically transformed into water soluble bicarbonates of calcium and magnesium, raising the total hardness as well as the alkalinity. One might expect the MgO to react exclusively with the excess CO2, eventually leaving a "skeleton" of inert CaCO3 behind. However, due to the porosity of pHlocrite™ filter grains, the CaCO3 component is active enough to join in the neutralising reaction. Many years of experience with dolomitic filter media as used for water treatment have confirmed the validity of the above equation in general.
It has been experienced that for each 10 mg/l excess CO2, approximately 18 mg/l alkalinity as CaCO3 (0.36 meq/l) is produced and the total hardness raised accordingly. The above equation generally holds true but, in the case of extremely soft waters, the activity of the CaCO3 component is accelerated, whereas in hard waters with a significant calcium concentration the CaCO3 activity slows down, giving a preference to the MgO component. It is obvious that there will be a consumption of pHlocrite™ almost entirely due to the amount of excess CO2 neutralised.
Following the above equation, a theoretical consumption of 1.06 parts of pHlocrite™ per part CO2 neutralised would result. However, as a small amount of material is lost during backwashing of the filter, it has become an accepted practice to operate with a consumption of 1.3 parts of pHlocrite™ per part of CO2 removed.
pHlocrite™ Grade Selection
pHlocrite™ Grade B - CaCO3 / MgO is the preferred grade for mineral acid neutralisation and corrosion control for hard waters containing excess CO2. Grade B is used in cases where higher activity is required for reduced reaction time or where it is employed mainly for pH correction in recirculated water systems such as swimming pools using acidic disinfectant when the make-up water is high in calcium. It is also very suitable for the neutralisation of mineral acid waste such as that discharged from ion exchange regeneration plants.
Analysis of pHlocrite™ B |
|||
Calcium as CaO |
28.6% |
||
Magnesium as MgO |
45.35% |
||
Silica as SiO2 |
3.13% |
||
Iron as Fe2O3 |
0.092% |
||
Alumina as Al2O3 |
1.155% |
||
Sulphur as SO3 |
0.073% |
||
Nickel as NiO |
<0.001% |
||
Copper as Cu |
<0.001% |
||
Manganese as Mn |
0.004% |
||
Cobalt as CoO |
<0.001% |
||
Loss on ignition (Total) |
22.86% |
||
Loss due to CO2 (Theoretical) |
22.5% |
||
Calcium carbonate equivalent to the calcium oxide |
51.194% |
||
pHlocrite™ Grade E - 99% CaCO3 /<1% MgO is a very high purity carboniferous limestone comparable and, superior to, other more expensive water treatment products on the market. It is crushed, milled, dried, screened and classified to produce an end product of exceptional purity and consistency.
Analysis of pHlocrite™ E |
|||
Calcium as CaO |
56.03% |
||
Magnesium as MgO |
<0.8% |
||
Silica as SiO2 |
<0.2% |
||
Iron as Fe2O3 |
<0.05% |
||
Alumina as Al2O3 |
0.1% |
||
Sulphur as SO3 |
<0.5% |
||
Nickel as NiO |
<0.1% |
||
Copper as Cu |
<0.1% |
||
Manganese as Mn |
<0.1% |
||
Cobalt as CoO |
<0.01% |
||
Loss on ignition (Total) |
43.60% |
||
Loss due to CO2 (Theoretical) |
- |
||
Calcium carbonate equivalent to the calcium oxide |
99.8% |
||
Detailed Analysis of pHlocrite™ Grades |
|||||
pHlocrite™ Grade B |
pHlocrite™ Grade E |
||||
Calcium as CaO |
28.6% |
56.03% |
|||
Magnesium as MgO |
45.35% |
<0.8% |
|||
Silica as SiO2 |
3.13% |
<0.2% |
|||
Iron as Fe2O3 |
0.092% |
<0.05% |
|||
Alumina as Al2O3 |
1.155% |
0.1% |
|||
Sulphur as SO3 |
0.073% |
<0.5% |
|||
Nickel as NiO |
<0.001% |
<0.1% |
|||
Copper as Cu |
<0.001% |
<0.1% |
|||
Manganese as Mn |
0.004% |
<0.1% |
|||
Cobalt as CoO |
<0.001% |
<0.01% |
|||
Loss on ignition (Total) |
22.86% |
43.60% |
|||
Loss due to CO2 (Theoretical) |
22.5% |
- |
|||
Calcium carbonate equivalent |
51.194% |
99.8% |
|||
Determination of Specific Amounts of pHlocrite™
1 part of pHlocrite™ Grade B neutralises approximately 1.2 parts of free CO2.
1m3 pHlocrite™ weighs approximately 1150 kilograms.
pHlocrite™ will need topping up when a maximum of 75% of the bed has been consumed.
A water containing 10mg/l (10 grams per m3) of CO2 will consume 6 grams of pHlocrite™ Grade B for every m3 of water passed through the pHlocrite™ bed and pro rata for any other level of CO2. If the flow rate through the unit is reasonably constant then it is a simple arithmetic operation to determine how long 75% of the bed will last at that flow rate.
Example
A 450mm diameter filter containing 0.13m3 of pHlocrite™ Grade B weighing around 150 kilograms will remove 135 kilograms (75% of 150 X 1.2) of CO2 before topping up is required. If the water to be treated contains 25 grams of free CO2 per m3 then around 3600m3 of water will be neutralised before top up is required.
At a flow rate of 2m3 per hour this will give a continuous filter run of 2800 hours or approximately 11 to 12 months assuming maximum flow rate for 8 hours per day.
Application of pHlocrite™ Dolomitic Filter Material
pHlocrite™ filter material is suitable for the anti-corrosion treatment of aggressive water characterised by low carbonate hardness or low pH resulting in a negative Saturation Index. Such waters may occur as:-
pHlocrite™ can be used in virtually any gravity or pressure filter tank and does not require additional equipment to that which is already standard on most conventional water filters. The filters should preferably be equipped with an air scour as part of the backwash system. Upflow or downflow systems may be used. Typical systems are illustrated here.
As the performance of pHlocrite™ is dependent only on the contact time with aggressive water, its rapid reaction rate means that filtration velocities can be selected within a wide range:- 5 - 30 m/h.
It is also applicable in the higher rate filters which are common in some swimming pool applications and in such cases, lower specific amounts are needed because the pool water is recirculated and filtration rates as high as 60 m/h. are tolerated.
Additional Features
Heavy metal removal is achieved due to the alkaline milieu on the surface of the pHlocrite™ grains, Fe2+, Fe3+, Mn2+ and Mn4+ ions (if present) are trapped in the form of their insoluble hydroxides and are removed from the grains during the next backwash. lead, cadmium, copper and zinc are also removed.
Trapping very fine suspended matter present in most waters is also achieved because of the angular surface of the pHlocrite™ grains which increases the surface area compared with more spherical grains.
It should also be noted that the purity of pHlocrite™ results in a virtually complete consumption of the filter material during operation and nothing is introduced to the water which is not present in all natural waters.
pHlocrite™ for pH and Alkalinity Control in Swimming Pool Water
Since 1982 when ICI Ltd withdrew chlorine gas for use in swimming pools, sodium and calcium hypochlorite have been used by most municipal pools for the disinfection of the water and, since both of these disinfectants are alkaline, acid is used to reduce the pH to the optimum value for pool water. A few public pools still use chlorine gas or liquid bromine and many smaller pools such as school and hotel pools use trichloro-isocyanurates for disinfection. All of these disinfectants are acidic when dissolved in water and require an alkali to maintain the pH in the correct range.
When chlorine gas dissolves in water, it hydrolyzes forming hydrochloric acid HCl and the weaker hypochlorous acid, HOCl.
Cl2 + H2O ![]() HCl + HOCl
HCl + HOCl
The acid from the continuous addition of chlorine gas to the pool water reacts with the alkalinity and is chemically transformed to CO2 and chloride.
2HCl + CaHCO3 ![]() CaCl2 + H2O + CO2
CaCl2 + H2O + CO2
This equation demonstrates that the decrease of alkalinity and pH is not only a function of chlorine gas but of all chemicals releasing hydrogen ions in water, e.g. the commonly used flocculant aluminium sulphate. Trichloro-isocyanurate releases HOCl and necessitates pH and alkalinity control, although, due to lower bather load and thus lower chlorine input, the effect is not as signifcant as in heavily loaded public pools.
The effect of chlorine gas on alkalinity and pH becomes conslderable when the initial alkalinity of the make-up water is lower than 200 ppm as CaCO3 (4 meq/l). Hence, pH control is of great importance in order to ensure the efficiency of the chlorine residual and the prevention of corrosion of all materials coming into contact with such an aggressive water.
pHlocrite™ features two advantages over other chemicals used for pH and alkalinity control (sodium carbonate, sodium hydrogen carbonate) automatic control of pH and alkalinity. Without any electronic measuring device, the pool water is kept well balanced.
SI = O ± 0.5
No problems with cloudy water due to improper dosing of soda ash or bicarbonate, improves removal of iron and or manganese possibly present in the make-up water.
pHlocrite™ is usually placed as a thin layer on top of the sand layer inside any conventional sand filter (gravity or pressure type). In respect to the necessary freeboard, a portion of the filter sand can be substituted without diminishing the efficiency of the filter, as pHlocrite™ is superior to sand due to the roughness of its grains.
Limitations
The application of pHlocrite™ dolomitic filter material is economical and feasible for corrosion control under the following conditions which should be carefully observed.
Carbonate hardness (often described as temporary hardness) which is the proportion of alkalinity covered by total hardness, should not exceed 180 mg/l as CaCO3 (3.6 meq/l).
Calcium hardness should not exceed 360 mg/l as CaCO3 (7.2 meq/l).
Non-carbonate or permanent hardness due mainly to calcium and /or magnesium chloride, sulphate and nitrate should not exceed 200mg/l.
Sodium as CaCO3 should not be greater than 60% of the sum of the calcium and magnesium as CaCO3.
To avoid unnecessarily hard final water, excess CO2 should normally not exceed 75 mg/l as CO2 but a sufficient amount of excess CO2 should be present in order to attain a minimum final calcium hardness to achieve corrosion control. If needed, the addition of pressurised CO2 should be considered but, usually, the addition of CO2 is confined to distillates and deionates where CO2 removal is inherent in the process and the calcium and alkalinity levels are practically zero.
Theoretically 10mg/l CO2 can produce nearly 23mg/l of hardness as CaCO3 but the actual amount produced would need to be established for each plant when commissioning and the required amount of calcium hardness increase would, of course, depend up on the level of calcium in the untreated water.
Where heavy metal removal is not the main requirement, iron and manganese contents should not exceed 2 ppm as Fe or 0.1 ppm as Mn. For higher levels, pre-treatment of the raw water is highly recommended in order to avoid reduction of neutralising capacity.
Continuity of filter load is desirable although short term deviations in the range of -30% and +10% are without significance. However, a total shutdown of a filter is always to be preferred to an underload operation. If a temporary underload situation becomes unavoidable, special attention must be paid to frequent and proper backwash in order to minimise the risk of clogging.
Other Methods for the Deposition of Calcium Carbonate Films
Like the dolomite process, other methods for deposition of CaCO3 films are based on the addition of alkaline calcium and/or an increase in pH until a state of CaCO3 saturation is reached and the conditions of a well-balanced water are met. Hydrated lime Ca(OH)2, mostly dosed to the water as a slurry but sometimes as a saturated solution, is widely used and marble or calcite chip filter beds to a lesser extent.
Whereas lime is usually the least cost chemical, it requires very accurate control in order to avoid over dosing and the required degree of saturation is seldom possible to achieve without the addition of CO2. Lime saturators or continuously stirred lime slurry preparation tanks are necessary and overall dosing complexity and costs make this the method of choice only for very large water treatment plants.
The required contact time in the latter method is rather lengthy, involving heavy investment in filter tanks to house the large amounts of marble needed to establish a well balanced water. However, the marble process is very safe, as no problems with overdosing are possible and since all of the increase in hardness is due to calcium it is sometimes preferred to the dolomite process when the raw water is extremely soft, does not contain significant amounts of iron or manganese and the slow reaction rate can be accommodated.
The dolomite process is highly recommended for application in small to medium-sized water works, lacking well trained personnel for the operation and maintenance of sophisticated dosing equipment.
Here are a few questions and enquiries we've had about our product
This Q&A page is regularly updated with enquiries we have received. They are included here as a guide to any questions you might have.
Glass media and cryptosporidium removal under swimming pool filtration
Q.I understand that you are interested in obtaining a copy of our Cryptosporidium Removal Under Swimming Pool Filtration Conditions Report. Am I correct in saying you produce pHlocrite™ Plus? It would be interesting to hear how our glass media can work in conjunction with yours?
Yes, we do produce pHlocrite™ 'B' and I think that pHlocrite™ would work very well with glass media but, unfortunately, not in swimming pools. pHlocrite™ neutralises the carbonic acid produced by carbon dioxide and thereby corrects the pH but the main acid contribution to swimming pools used to be the chlorine gas used for disinfection which is quite acidic.
Nowadays, most municipal pools use sodium hypochlorite for disinfection which being strongly alkaline does not work at all well with pHlocrite™ or any other dolomitic limestone. Some domestic pools use Trichlor for disinfection which is acidic and therefor suitable for use with pHlocrite™ but the usage is very small and not of great interest especially since we do not sell in small quantities but only in tonnes and multiples of tonnes. Most of our output goes to the various companies who make and/or market small fibre glass filters for the treatment of water for single households or groups of households with their own boreholes and these filters almost invariably have sand support for the pHlocrite™ which I am sure could very easily be replaced with glass media.
We also sell a few tonnes a year to the big distilleries who use it for neutralising the excess mineral acid from the regeneration of their deionisers.
There has been a great deal of interest in glass media in recent years and claims are regularly made with regard to its superiority over sand but I have yet to see the publication of any studies or measured trials comparing the effluents of the two when being fed with the same source water.
I guess that I am a bit biased when looking at some of the claims that are made for filtration systems without actual measurements, so you can understand my interest in your report on the removal of cryptosporidia by swimming pool filters using glass filter media which sounds like just the sort of study that I have been looking for.
Firstly thanks for the information regarding pHlocrite™, I must be honest and say I had not heard of it before your enquiry. However I know Steve at Specialist Aggregates quite well and found your product via his site. Whilst our main market is currently swimming pool filtration, we are constantly expanding into other areas. We obviously have DWI Reg31 approval for potable water which was assisted by supply to Affinity's Egham WTW and the test results of Mark Watts at WRc PLC.
The Crypto report was produced under guidance of PWTAG and shows Enviro glasmedia to be equal to silica sand and a rival glass media (AFM). The test served a purpose at that time in order for us to supply the Welsh National Pool and Commonwealth Pool in Glasgow.
There are some big claims made about glass filter media, however very few can back up those claims. We know that we produce a suitable product and undertake the necessary research; however it is this research which others replicate and embellish. One point I will make is that we believe honesty is the best policy; therefore all our testing is done via third parties - this way we can provide lab results for any claims we make.
Obviously working together with pHlocrite™ on the home water systems would be ideal and something which I am sure would appeal as a sustainable solution for households.
Similarly, it would be good to know how Watts & Associates could further form a link with DMS Enviro. We are currently seeking market openings into power stations, food processing facilities and mineral water boreholes. Another area we are concentrating on is slow sand filtration in tandem to RGF.
The WRc report is exactly the sort of thing I have been looking for thank you and is all the better for being carried out by my namesake, although I do not think that Mark Watts is related to me. I did know about Dr Crolls' work at Swansea from my long time contact with PWTAG when I was involved in the writing of the filtration section of their swimming pool manual but it does not cover the necessary ground like the WRc report. The WRc at Medmenham also did some work on pHlocrite™ many years ago before being privatised when they were quite excited by its possible use for colour removal from peaty waters.
Yourselves and pHlocrite™ Ltd working together sounds great but at the moment we produce and sell only pHlocrite™ and not the filtration systems. The people that you need to contact are the filter manufacturers who specify the media content of their filtration systems such as Euraqua U.K.(Ferex Ltd) of Hitchin and Shakesby and Sons of Norwich because although I can influence them in their choice of dolomitic materials I do not think that they would be interested in my views on support media although I could endorse any approach made by yourselves.
You will appreciate that although these filter manufacturers use pHlocrite™ in the pH correction filters they also market straight sand filters for particulate matter removal and quite often use very expensive support media in their iron removal filters which might easily be replaced by glass media.
Flow rate and retention time in filter bed ?
Q.I just have a quick question, regarding pHlocrite™ quantities, if that's OK. We'd like to know what quantity we should be using, the details are:
1. Private bore-hole water supply, Iron-manganese ration of 1:2 (1ppm and 2ppm).
2. Filtration designed around the Triplex system (with aeration vortex)
3. water pH c.6.23.
4. Based on point 1, I think we would need to reach a pH of c.8.3 to remove the manganese
So my question is, how much pHlocrite™ (weight) do with need to mix with birm (or other) to achieve the above? I have only just started to look into this in depth, we have been using a local company since we moved here and the water has always been terrible. After another invoice, I started to look into this and realise that they have never used a pH corrective media- I think this is the cause of our problems.
As a side note, I think this product would also be very useful for our sites under irrigation, that struggle with maintaining high pH levels.
Basically, it is not a question of how much dolomite to mix with iron removal mineral but more a question of flow rate and therefore retention time in the beds. When using dolomite (pHlocrite™) for iron and manganese removal you should not exceed a flow velocity of 10m/h and pHlocrite™ Grade 'B' should be used to ensure that the pH reaches at least 8.3.
We prefer using manganese dioxide as a catalyst rather than birm to remove the manganese for a variety of reasons but before we can advise you further we need to know the alkalinity and the calcium hardness of your raw water and the filtration velocity. Does the aeration vortex introduce enough oxygen to oxidise the iron and manganese? You can determine this by checking the oxygen level of the treated water. You may need to chlorinate the raw water to obtain optimum iron and manganese removal if the oxygen does not do the job on its own, especially in the presence of organics such as fulvic acid.
Most borehole waters are low in organics but it is worth checking since the failure of simple filtration systems to remove iron and manganese is very often due to the presence of organics which seriously inhibit the oxidation process.
Many thanks for this, its really very helpful. I've dug out our last bore-hole water results and they are:
Alkalinity = 63.6 mg/l HCO3
Hardness = 16.4712 mg/l Ca
Turbidity = 11.70 NTU
Iron = 1070.0 ug/l Fe
Manganese = 2050.0 ug/l Mn
Filtration velocity = I'm not sure how to find this measurement.
Thank you for the advice on Manganese dioxide (would this be better than using Filox-R?). Thank you also for the advice on DO, it is also something we will look into along with organics.
So it sounds like we should have a mix of pHlocrite™ Grade 'B', Manganese dioxide and a filter media. With the above in mind, are you able to estimate how much of each media would be sufficient?
I thought it would be wise to point out that the water is flowing with a very strong orange colour which I assume means it is well oxygenated. From our hot tank, the water comes out clearer as the water has had time to settle.
The orange colour is obviously precipitated iron oxide so there must have been enough oxygen to have produced that. It would be a good idea to take that iron oxide out with a simple sand filter before putting it through the catalysed dolomite, simply to reduce the load on the latter. Filox-R is probably a trade name for MnO2.
To calculate the filtration velocity I simply need to know the flow rate of the water and the diameter of the filter.
Purely as a matter of interest, who supplied your filter? (Probably Euraqua or Ferex Ltd). Did they know how much iron and manganese were in the water ?
Great, the figures are:
Flow rate: 42litres/minute. Measured as water running into the house, collected as running through 3 big taps at full blast. Noticeable pressure reduction, with each additional tap switched on (after tap 1).
Diameter of the filter vessel: 370mm (approx height 1890mm)
Specified quantity of gravel: 2x 25kg bags
Specified quantity of 'triplex media': 3.5 cu.ft
The data sheet we have for the system states the manufacturer as Euraqua. However, its all been done by a local company. They did our full water tests and had the results, but still decided to refill the filter tank with solely Filox media. I naively assumed they were experts and would do what was best.
It will be very reliving when we can bath the kids in clear water and use the washing machine again!
42 litres/min equals 2.52m3/h. According to the Euraqua data sheet your particular filter is rated at 2.3m3/h. Assuming that you do not normally have three faucets open at any one time we can assume that your normal flow rate will be around 1.5m3/h. If you divide the flow rate of your filter in m3/h. by the area in m2 you get the filtration velocity. The area of your filter is about 0.11m2 so the filtration velocity at 2.3m3/h appears to be 21m/h and at 1.5m3/h around 13.6m/h.
With the amount of iron and manganese in your water I would not rate an Fe/Mn filter above an absolute maximum of 10m/h and I would definitely have a sand filter after the aeration stage to remove the easily precipitated iron before putting the water through the iron removal filter but the makers presumably think otherwise. I would also pump the well water at a constant rate to an overhead tank and take the distribution from the tank so that the variations in flow rate due to the varying number of taps that might be open would not occur.
pHlocrite™ grades and topping up ?
Q. In various places we have pH correction vessels for varying reasons and at service periods the correction media needs to be replaced.
On Thursday of last week I topped up such a vessel with a media lable "pHlocrite™" and in a box next to the word "Grade" the letter "B" had clearly been written. I've seen many dozens of such bags, but I have never seen one with "A" written in the box. However, in a conversation I have had since, I was told that there is no way that it could have been Grade B I had been using as it is too strong and used only for the treatment of sewage. To use it on groundwater at a pH of, say, 6.3 would be to "send it through the roof". In this particular application we are ideally looking for a pH of 8 to 8.5.... and I'm fairly certain I haven't witnessed such a pH using "B" grade pHlocrite™.
This leads me to the conclusion that someone has been given some duff information that does not correspond with what I think I know. If the bag says "B" grade pHlocrite™ then it is my view that it must in fact be "B" grade. If we used "A" grade then we would be at a disadvantage due to a longer reaction time and thus a lower pH at the passing into the following vessel. Given that the whole process has been designed to remove Manganese from water in a single pass, I would have thought that the reaction time of grade "B" would have been factored in as desirable and we would have been supplied with that instead of the slower acting grade "A".
Please just put my mind to rest, or explain why my logic is out of kilter?
First of all we don't supply Grade A anymore (it can be produced if required by mixing Grade B and Grade E). You are using the right grade (Grade B) for manganese removal. It is most certainly not designed for sewage. There is not much difference between the reaction rates of the two grades but when treating very soft waters we now normally recommend a Grade B/E 50/50 mix because it has a higher calcium content which is preferred with these low calcium waters to facilitate obtaining the necessary slightly positive Langelier Saturation Index for corrosion control without getting the pH too high. When there is sufficient calcium in the water to be treated and there is a lot of carbon dioxide then we recommend Grade B.
Grade B is used extensively in the distilling industry for treating the waste acid from the regeneration sequence of their demineralisation plants because it is stipulated by the authorities for this purpose. Since this treated acid effluent is discharged to the sewerage system I suppose this is where the suggestion that Grade B is for sewage treatment comes from.
I have suggested to the distillers that they might like to pay for their pHlocrite™ supplies with cases of whisky but there seems to be a problem with the Customs and Revenue people with this proposed barter
Remineralisation as well as corrosion prevention ?
Q.I am a ventilation & building services engineer, working in the nuclear industry. We are increasingly being asked to re-mineralise steam condensate from steam heating coils.
Currently I am working on an urgent project where I have approximately 650 ltrs/hour of condensate that I need to re-mineralise prior to its discharge into the ground water drains. The condensate will be cooled to below 42°C prior to re-mineralisation, the only slight problem is that the discharge from the facility is only approximately 400mm (Therefore for large tanks I may need to add a pump).
Could you please advise me on the products you design / supply which could meet the above requirements?
We manufacture granulated dolomitic limestone and a Grade'B/E' 50/50 mix is normally recommended for remineralisation. Suitable wound fibre glass vessels are supplied by Euraqua U.K. at Hitchin. Their 12 inch diameter Filter No.1248 would be more than adequate for 650 litres/hour. These units can be gravity fed as there is very little headloss through them but 400mm is a bit low and you will probably need a small pump.
I am a bit puzzled for the need to remineralise before discharge to drain unless the drainage system is metal and the aim is simple corrosion prevention. Remineralisation of distillates and condensates is normally for re-use as well as corrosion prevention, but to achieve an optimum level of calcium bicarbonate in the water for re-use rather than simple pH adjustment, you need some carbon dioxide in the influent and there is unlikely to be much carbonate dioxide in the condensate at temperatures approaching 42°C. However, I would think that simple pH correction would be sufficient for discharge to drain.
Borehole Water Treatment
Q.We started to have a metallic taste and cabbage - like odour in our water post treatment. I phoned pHlocrite™ about the problem and they expressed the wish to be informed about any progress which I made.
Our plant has the following stages in this order:-
A. Aeration via a Mazzei 584 venturi injector.
B. EN aeration mixing tank (Vessel size 1354).
C. In-line pressure buffer vessel.
D. Multi media filter - gravel, two grades of sand, anthracite. (Vessel size 1665). Set with10 minutes backwash.
E. pHlocrite™ (Vessel size 1665). Set with 10 minutes backwash.
F. Filox (Vessel size 1354). Set with 10 minutes backwash.
G. UV steriliser.
Backwash flow rates were found for stages D,E and F to be well below specification (spec. is 3m3 / hour).
During the stages 1 to 6 below, the Environment Agency did an analysis ex kitchen tap which showed that manganese was illegally above the Drinking Water Standards at 2.8 mg / l. Iron was satisfactory at 0.094 mg. / l. So despite the fact that at that time I had got the iron under control then manganese was still a major issue.
Several remedial steps were taken:-
1. 20", 5 micron filter added.
2. 20", 20 micron carbon filter added.
3. EN aeration tank pressure set at circa 30 to 45 psi. Aeration is visually and audibly occurring well.
4. Backwash times doubled from 10 to 20 minutes on all tanks.
5. Size of backwash flow restrictors increased from 9 mm. / 9.5 mm. to 11mm. / 12mm.
6. A piece of stainless steel swarfe was found blocking the Mazzei injector.
Steps 1 and 2 immediately improved the palate.
Backwash flows all increased but were still nowhere near specification. The best achieved were all from 2.2 to 2.5m3 / hour. Backwashes for Multi Media and pHlocrite™ showed thick ferric oxide right though and did not clear.
When the plant was serviced the EN aeration tank contained quite a lot of ferric oxide. The Multi Media Filter contained even more. However the pHlocrite™ contained even more and about half of it had been converted to a brown concrete like mass. The time taken to clear this out prevented the Filox tank being serviced.
The Multi Media Filter and the pHlocrite™ were renewed. A 9.5 mm backwash restrictor was used on one tank but the specified flow rate was again not achieved and it was then replaced by an 11mm. restrictor. Backwashes for Multi Media and pHlocrite were set at 10 minutes. Filox was set at 20 minutes.
pH results:-
Alcontrol when borehole first sunk in 2010 - 6.6
Severn and Trent ex borehole in 2011 - 6.96
Severn and Trent post treatment in 2011 - 7.86
Fellside Barn (Hach test strip) post treatment in 2013 - 7 / 8
Iron results ex borehole (micrograms per litre)
Alcontrol in 2010 - 4330
Severn and Trent (filtered) in 2011 - 4590
Severn and Trent (unfiltered) in 2011 - 8280
Fellside Barn (Hach test strip) post treatment in 2013 - 0.1 / 0.15 milligrams per litre.
So treatment is now working well. Though manganese is still unknown and test strips are awaited ex U.S.A.
Backwashes have been checked:-
Multi Media at 3.5m3 / hour with no overflow of media. No ferric oxide obviously visible
pHlocrite™ at 3.4m3 / hour with no overflow of media. Heavy ferric oxide for about half initial backwash then clearing.
Filox at 2.8m3 / hour (should be 3). No ferric oxide visible.
Comment:-
I have read the two documents attached. Clearly the rate of iron oxidation is very significantly slower below pH 7 than it is above 7. For manganese, I gather pH in excess of circa 8 is essential. It would seem to me that the correct configuration to optimise the process should be:-
A. pHlocrite™.
B. Air injection.
C. EN aeration mixing tank.
D. In- line pressure buffer vessel.
E. Multi Media filtration.
F. Filox.
G. UV
Backwash regimes would also need to revision. Flow restrictors of 9 mm / 9.5 mm would seem quite inadequate. Times also need to be reconsidered especially since pHlocrite™ has an obvious affinity for iron and it will merit a much longer backwash.
Even allowing for the sophisticated treatment system, I think that you have done extraordinarily well to get the iron down to an acceptable level when considering the very high amounts of iron and manganese in the raw water but I do not think that putting the pH correction vessel first in the line up will help with manganese removal and unless the borehole water is completely free of oxygen it could make things worse.
I would like to calculate the maximum pH that you could expect to achieve with the existing set up and for that I need to know the pH, alkalinity and calcium hardness of the raw water at present. You are correct in thinking that you probably need the pH to be higher for manganese removal because the rate of oxidisation of the unfilterable soluble manganese to the filterable insoluble form is pH dependent. You may be able to achieve oxidisation at the lower pH by running the Filox stage slower which would mean putting in a larger Filox unit or adding an extra unit in parallel to the existing unit. The dual unit approach is preferred because they can be backwashed individually at a lower rate than a single large unit.
It would appear from your diagram that the backwash of each vessel is made from the effluent of each preceding vessel and not from a dedicated wash water tank holding final treated water. Since units B and C are not designed to remove iron but simply convert it to the insoluble form, and the service flow rate through the plant has to be doubled to obtain the wash rate it is not surprising that the backwash effluent from units D and E did not clear since they will normally remove very little iron in backwash mode.
It is surprising, therefore, that after the air injector was made to work correctly, you were able to backwash units B and C and eventually obtain a clear backwash effluent, unless the increased air injection enabled Units B and C to retain a lot of the iron and/or Units D and E are now removing iron in the backwash mode because more of the iron has been oxidised by the increased air.
When you have provided me with the raw water figures I will come back to you with a couple of ideas that I think could reduce the manganese to an acceptable level.
I have been doing some trials. Basically, what I have found so far is that the specified backwash flow rates are too low. That recommended for all my vessels (1665's for Multimedia and pHlocrite™ and 1353 for Filox) are all 3m3 per hour. I have been experimenting with larger orifice sizes and backwash times. In general, from calculation, it is better with high iron content to increase the orifice size rather than extend backwash time. Essentially this is because one gets fluidisation of the bed with increased orifice size. You made the point about backwashing with the effluent of a previous stage. If you do this without fluidisation all that happens is a build up of filtered iron oxide which can then move to the next stage when normal filtration resumes.
I have actually seen this occur. Multimedia backwashed at 3.1m3 per hour was clear right through. I then increased the orifice size and backwashed at 3.8m3 per hour. The water was thick brown for several minutes and then settled to a whisky colour. So retained iron oxide was washed out when the bed was fluidised and the rest of the backwash water contained newly converted iron oxide which was not retained on the bed.
The pHlocrite™ responded to the increased Multimedia backwash flow rate. Brown at first and then clear. I increased the orifice size to the same as that for the Multimedia (12mm) and got 3.41m3 per hour and it was clear all though. I want to see what effect a 13 mm orifice would have but am proceeding cautiously. Fluidisation is essential but so also is not losing all the media down the drain!!!
I have had mixed luck with the Filox. As a result of the work on the Multimedia it went up to 2m3 per hour. The Filox 1354 is fitted with a Fleck 2150 head. The backwash diameter is limited to the size of the backwash outlet (8mm). Unfortunately I broke this and had to stick back together with araldite. I managed to get a spare. However this gave me the opportunity to drill out the araldited one to 9mm. Guess what - the flow went up to 2.99m3 per hour. So obviously with that flow limitation the Fleck 2150 is useless for backwashing a 1354 Filox tank.
I have ordered a Fleck 2750. The 2750 does not have the built in 8mm flow restriction so I can try that at 12mm and see what happens.
I have also just taken delivery of some manganese test strips. I could only get them through one company in Europe and they had to get them from the USA. They contain KCN which might explain their unavailability here!
So, when I get the new Fleck I will see what flow rate I can get through. Following that I will do some Fe, Mn and pH's and then check it all out again and write it up.
At the moment it seems that lack of fluidisation, most especially of the Multimedia, is the cause of a lot of problems. Increased orifice size may well be the answer. Increased backwash times is not and may even make the problems worse.
There is one thing which I perhaps ought to add. That is the size of the flow restrictors originally supplied. The 2 x 2750 and the 1 x 2510 all had flow restrictors of 9mm to 9.5 mm. As you will realise that is no good for the 2510 when the outlet from the head has a diameter of only 8mm anyway. So according to my results, the installation was doomed to early failure from the very beginning and the piece of metal swarfe partially blocking the air intake ensured this failure.
I would like to know what you think. This may have implications for your product in this sort of scenario. As I wrote before, when the equipment was serviced, the pHlocrite™ tank was set like concrete and most of the operators time was spent trying to get it out.
It is a bit difficult for me to comment adversely on a specific supplier's filter system design when they are pHlocrite™ customers but you are quite right in thinking that low wash rates cannot be compensated for by extending the wash duration. That is basic filtration engineering.
The wash rate for deep bed filters must be sufficient to fluidise the bed and is the minimum rate that should be used. Velocities beyond the minimum fluidisation rate can be beneficial and some filter makers specify rates that will result in a bed expansion of 10% or 20% but the benefits are very seldom worth the extra cost. This is because washing efficiency is governed by the velocity of the water passing individual filter medium grains which is maximum at the onset of fluidisation and remains the same with increased overall wash flow velocity because the flow increase beyond the onset of fluidisation causes the filter medium grains to move further apart so that the velocity passing individual grains is unchanged.
The fluidisation rate for various filter media is a function of the viscosity of the water (which varies with temperature), the specific gravity of the filter medium, the size and the shape (angular or rounded) of the filter medium.
I have attached a graph showing the fluidisation velocity in mm/sec for sand and for anthracite @ 15°C. If you multiply the mm/sec figure by 3.6 you get the velocity in m/h and if you multiply that figure by the area of the filter you get the flow rate in m3 /h. Since most filter media are polysized you have to base the fluidisation rate on the largest grains in the range so that when calculating for 16/25 sand which has a size range of 0.6 to 1.0mm you should base your calculation on the 1.0mm size sand grains. For your 1665 sand /anthracite filter (area 0.13m3 ) the minimum fluidisation velocity works out at a minimum 2.25m3 /h for the sand which would also be adequate for the anthracite because although it is larger particle sized it has a lower specific gravity. Obviously, this means that your wash rate of 3m3 /h will be ideal for that filter.
pHlocrite™ has the same specific gravity as sand so you could use the sand data to establish the fluidisation velocity, which if you take the largest size grains in the pHlocrite™ (Size Range 1 - 4mm) you get a fluidisation rate of 18.5 m3 /h but, fortunately, when calculating for a soluble medium you can take the median size of 1.8mm for your calculation which gives a backwash rate of 5m3 /h. which would prevent the cementing problem that you had at the original rate.
I do not have data for Filox so I cannot calculate the fluidisation rate.
If your plant had been designed for pH correction and removal of small amounts of iron and manganese I would not see much wrong with the design but with the amount of iron and manganese in your water I would definitely have specified a wash water tank and backwash pump so that the filters could be backwashed with clean water.
'Clumping' & Calcified Heating Elements?
Q.I have a couple of questions about the use of your product and hope that you can help. We use bore hole water, and about 18mths ago the water became acidic around pH 6.25. We therefore devised a filtering system using your product and it has mostly served us well.
We use about 4500 litres/day and feed the water through a barrel (57cm diameter x 62cm depth of phlocrite™ - quantity 175kg pHlocrite™ ). The delivery rate through the filter is max 15 litres/min. We backwash every 2 weeks but we have noticed that over a period of months the pHlocrite™ clumps together and becomes ineffective ..
So my questions
1. is 175kg in the barrel set up about the right amount and how long would you expect it to last?
2. what can we do to stop the clumping?
The main reason for 'clumping' is low backwash velocity. Our minimum recommended backwash velocity is 30m/h which for a 57cm diameter vessel (area =0.255m2) that would be a flow rate of around 7.5m3/h. Your current filtration flow rate is 15 litres/minute ( 0.9m3/h) giving a velocity of 3.6m/h in a 57cm diameter vessel which is acceptable as a filtration velocity but, obviously, too low for efficient backwashing.
It is also critical that the backwash flow is applied evenly through a well designed flow distribution system otherwise you get channelling through the filter medium bed which leaves large parts of the bed unfluidised and, therefore, not washed and subject to 'clumping'. Backwashing should not be carried out less than once per week.
For a reasonable bed depth, filtration velocities up to 20 m/h are acceptable in down flow filter units so that a much smaller diameter vessel could be used for your flow rate of 15 litres/minute which would
result in the need for a much lower backwash rate. A 25 cm diameter vessel could be run in filtration mode at 15 litres/minute and require a backwash rate of only 15 litres/minute.
Most filter suppliers using dolomitic media for pH correction use them in up-flow mode with a maximum filtration velocity of 12m/h so that you would then need a 30cm diameter vessel for your filtering flow rate of 15 litres/minute and a wash flow rate of around 22 litres/minute.
The amount of pHlocrite™ used is dependent upon the carbon dioxide content of the raw water and the filter flow rate (4500 litres per day). For a carbon dioxide content of say 20mg/l you would be neutralising 90 grams of carbon dioxide per day which would consume 75 grams of pHlocrite™ Grade 'B' per day which would require topping up the filter bed every 4 or 5 years years.(75% of 175kg X 1000 divided by 75 divided by 365 = Years )
Allowing for around 10% wastage due to fines removed during the backwash sequences. The attached Technical Note No.2 will enable you to calculate your actual projected usage when you know the true amount of carbon dioxide in the raw water.
We trust that we have answered all your queries but please feel free to contact us again if you require any further information.
Did I thank you for your very helpful reply - I really appreciated it and have taken steps to improve our system.... Come March we will have been filtering our water for two years but because of clumping the pH has mostly been at 6.5 ( which I now expect to improve in the future ) We did have a couple of pH readings - one 7.7 and the other 7.2 after we changed the filter medium.
I'm giving you this background because I'm wondering whether you can shed any light on why we are having a problem with heating elements becoming calcified. We have had three new, as in 2 year old, elements failing - and when taken out one was found to be so calcified it was difficult to get out of the cyclinder.
I can't make sense of having a mainly acid water with such calcification .. but it does seem to have occurred in the timescale of filtering our water.
Calcification of heating elements can only occur if the treated water is high in calcium bicarbonate. For the treated water to have a high calcium bicarbonate level then either the raw water is high in calcium or the carbon dioxide content of the raw water is high enough to dissolve sufficient calcium from your dolomite filter to cause scaling when heated. It is not uncommon for a borehole water to have a low pH but still have a lot of dissolved calcium bicarbonate due to a very high carbon dioxide content but I would not expect to find this in waters in your area so I think that we have to assume that your raw water contains a lot of carbon dioxide.
Can you have the CO2 level checked by your local water supplier or laboratory. I would do it for you but the test needs to be done at the borehole because excess CO2 will be lost during transit.
Try checking the pH of the raw water and then re-checking the pH after vigorously shaking the sample for a couple of minutes before e-mailing me with the results. If these results reveal a high carbon dioxide content you will have to think about installing a simple step aerator to remove the excess before passing through your filter.
Following up your suggestion of comparing a still with a shaken water sample - the still pH was 5.5 and after shaking dropped to 4.5. Would this be what you'd expect ? as an indication of acidification resulting from dissolved carbon dioxide.
No! I would not expect the pH to drop, it should have risen. In water with a pH greater than 3.6, the acidity is always due to dissolved carbon dioxide so that if you shake a sample, some of the carbon dioxide will be released into the atmosphere and the pH will rise.
What is your method for measuring the pH ?
Yes I was surprised.. I'm getting someone here to do the testing using Wide range water testing pH strips from Simplex Health... I will test again and ask someone else to take a readings...also we are looking for someone local with expertise on Borehole water chemical states.
I'll get back to you next week with more results hopefully.
Once again I appreciate your help with this - it's very supportive as we try to understand and solve our water problems.
pHlocrite™ pH Control ?
Q.I have incoming water ph 6.1. Have been looking at a 10*54 inch pH filter tank with pHlocrite™ "B" media. Can you advise if this product stops the pH at 7.2 like juraperle/calcite - or does it continue to react in the water and raise ph to over 7?
Plain calcite is much slower reacting than dolomite so the water is unlikely to reach equilibrium at the filtration rates normally allowed for by most of the companies selling pH correction filters.
Dolomitic limestone reacts much faster than plain calcite so will normally allow the water to reach equilibrium. Most waters with low pH due to dissolved carbon dioxide will have a pH of saturation of around 8.4 so that if you reach 7.2 with plain calcite you will probably reach to around 8.4 with dolomite.
I am enclosing the calculating method for determining the Saturation Index for any water.
pHlocrite™ pH Control ?
Q.I have incoming water ph 6.1. Have been looking at a 10*54 inch pH filter tank with pHlocrite™ "B' media. Can you advise if this product stops the pH at 7.2 like juraperle/calcite - or does it continue to react in the water and raise ph to over 7?
Plain calcite is much slower reacting than dolomite so the water is unlikely to reach equilibrium at the filtration rates normally allowed for by most of the companies selling pH correction filters.
Dolomitic limestone reacts much faster than plain calcite so will normally allow the water to reach equilibrium. Most waters with low pH due to dissolved carbon dioxide will have a pH of saturation of around 8.4 so that if you reach 7.2 with plain calcite you will probably reach to around 8.4 with dolomite.
I am enclosing the calculating method for determining the Saturation Index for any water.
Please contact us for pricing and availability.
We usually transport both grades from our Cornwall depot by the tonne for maximum economy. However, we are happy to quote for smaller deliveries of 20kg or more.
We guarantee our factory prices are the lowest in the UK and only alter with the cost of our raw materials and transportation.
























